Stock Management with LIMS
Keep lab inventory accurate, compliant and audit-ready with real-time stock visibility, automated tracking and complete batch-level control.
Clear stock visibility, without the complexity
Labs need confidence in their inventory to run smoothly. Manual processes make stock harder to track, easier to waste and difficult to plan around. We’ve built stock management tools that centralise everything, keeping inventory accurate and visible in real time – helping lab teams stay organised, reduce waste and keep testing on track.
Smarter stock control for every lab
Manage stock with confidence across your entire lab. LabHQ gives teams a clear view of every item and batch in one place, so they can plan accurately, avoid disruption and maintain consistent, audit-ready processes.
.svg)
Centralised Inventory Records
Store all stock item templates, categories, units, quantities and batch information in one organised system.
.svg)
Automated Tracking & Alerts
Track stock levels automatically and receive clear low-stock indicators using thresholds set per item.
.svg)
Batch Lifecycle Visibility
See every batch from receipt to approval, usage in testing, expiry and disposal - with full traceability at each stage.
.svg)
Real-Time Usage & Audit Trails
Review every consumption, disposal, correction or testing-related usage event with complete audit history.
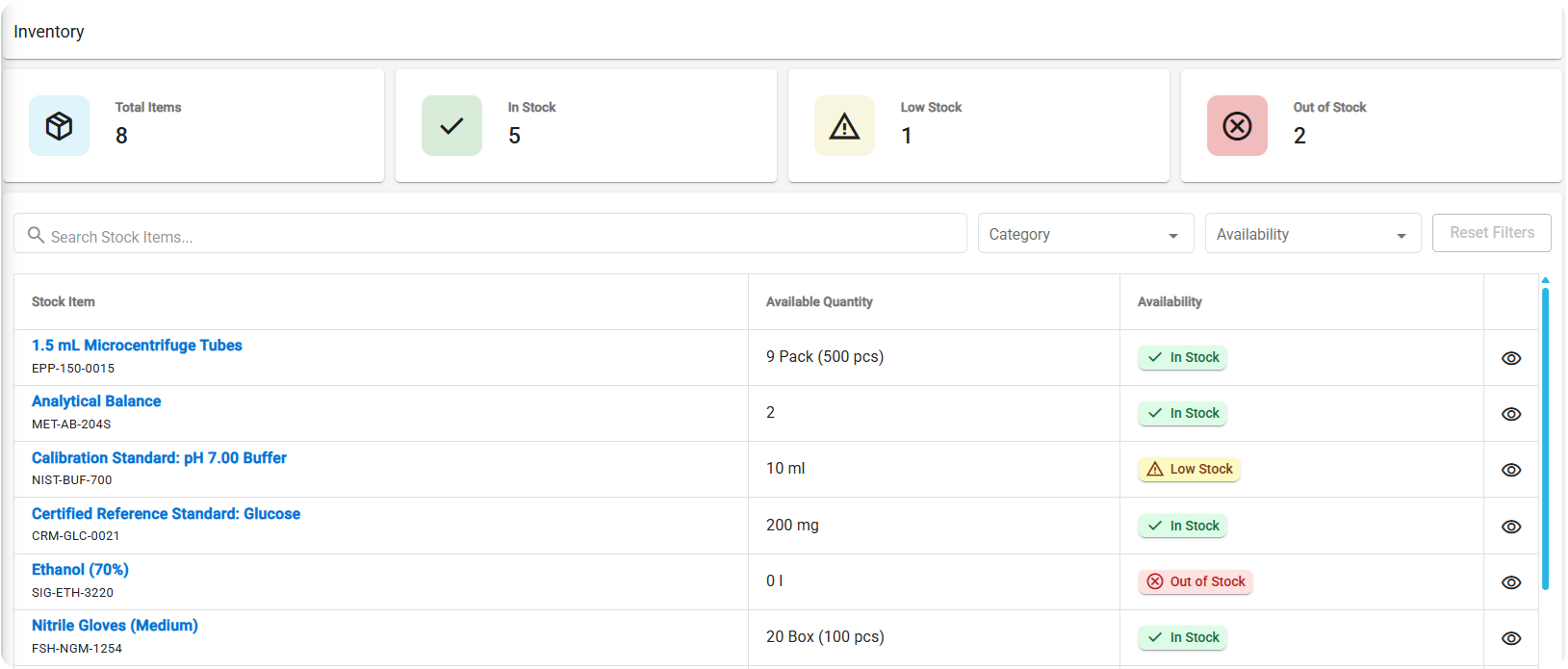
Real-time Inventory Dashboard
See accurate stock levels at a glance, including total usable quantities across all active batches
Stock Item Templates
Standardise setup with predefined templates for item names, codes, units, thresholds and categories.
Expired & Expiring Stock Visibility
Quickly identify expired stock and due to expire within 30 days to reduce waste.
Batch Status Tracking
Track each batch through testing, approval and rejection for complete lifecycle visibility.
Automated QC Testing for Incoming Stock
Trigger QC workflows automatically when materials require testing, ensuring only approved stock enters use.
Full Stock Usage Tracking
Log every consumption, disposal and correction event with reasons for complete traceability.
"LabHQ really impressed us. They spoke our language and genuinely understood our needs from the beginning."
Comply with regulatory standards for…
FDA 21 CFR Part 11
Stay compliant with FDA 21 CFR Part 11 by maintaining secure, trackable electronic records and signatures.
MHRA & HIPAA
Meet MHRA guidelines and protect patient data with HIPAA compliance for data safety.
ISO 17025
Support ISO 17025 certified with validated testing methods and accurate, traceable lab results.
GMP & GLP
Adhere to Good Manufacturing Practices (GMP) and Good Laboratory Practices (GLP) to ensure safe products through strong quality control (QC), clear documentation, and consistent, reliable non-clinical lab data.
HPRA
Stick to Health Products Regulatory Authority (HPRA) standards for quality and safety in lab testing.
ISO 9001
Think of it as the blueprint for quality management. ISO 9001 ensures consistent, high-quality outputs and helps maintain trust with customers and stakeholders.
Stay ahead in lab operations
Regulatory updates, efficiency tips, and product news for busy QC labs.
Monthly emails. Unsubscribe anytime.
FAQ
How does LabHQ track stock levels automatically?
LabHQ updates stock levels automatically as stock is received into the lab, used in testing, adjusted, or disposed of. Stock is tracked by batch number and LabHQ monitors quantities and expiry dates, giving labs real-time stock visibility.
Does LabHQ prevent the use of expired or unapproved stock?
LabHQ flags expired stock and stock due to expire in the next 30 days. It also tracks approval status, helping prevent the use of expired, rejected, or unapproved materials.
How does LabHQ stock management help reduce inventory costs?
LabHQ helps labs reduce inventory costs by improving visibility and control. Labs can identify expired or wasted stock, avoid over-ordering, and set minimum thresholds to prevent stockouts.
How does LabHQ stock management support regulatory compliance?
LabHQ provides a complete audit trail for all stock activity, including batch history, usage, disposal, and approval status. This helps maintain compliant, traceable inventory records and prevents the use of unapproved or expired stock.
Can stock be linked to specific tests or methods and integrated with other areas of LabHQ?
Yes. Stock can be linked to LabHQ test methods with the standard quantities required for testing according to your method procedures, so stock usage is automatically recorded during testing. Stock management is fully integrated with LabHQ’s QC lifecycle, allowing usage to be tracked against testing activity in one system.


.webp)






